Business
H-1B: Man spent $8,000 on flights to get back to the US after visa fears

Rohan Mehta – not his real name – spent over $8,000 (£5,900) on flights in his scramble to get back to the US ahead of a deadline that would dramatically increase visa fees for some.
He had been in Nagpur, India for the anniversary of his father’s death before he cut his trip short.
On Friday, President Donald Trump signed an executive order adding a $100,000 (£74,000) fee for applicants to the visa programme for skilled foreign workers which US-based companies would have to pay.
Companies and immigration lawyers had already advised those on the H-1B visa who were outside the US to return before the order came into force Sunday.
A day later, the White House clarified it would be a one-time fee and would not apply to current visa holders, but it was too late for some.
Workers from India receive by far the most skilled visas in the programme, at more than 70% of the 85,000 issued each year.
Despite the clarification posted on X by White House Press Secretary Karoline Leavitt, concern and confusion had already spread.
The BBC spoke to many H-1B visa holders from India.
Many have been working in the US for decades.
None wanted to be identified as they were not authorised by their employers. Many refused to speak to us entirely.
Rohan Mehta, a software professional, has lived in the US with his family for 11 years but had travelled to Nagpur at the beginning of the month to see relatives commemorating his father’s death.
But on 20 September, he said he feared he would not be able to return to his home if he did not get back before the deadline.
He spent over $8,000 (£5,900) in eight hours booking and rebooking return flights to the US.
“I booked multiple options because most were cutting it very close,” he said just after he had boarded a Virgin Atlantic flight from Mumbai to John F. Kennedy International Airport.
“Even if there was a slight delay, I’d have missed the deadline.”
In its clarification, the White House said the new fee, which is more than 60 times the amount currently charged, would not be enforced until the next round of visa applications was approved.
Rohan Mehta described the last few days as “traumatic” adding he was glad his wife and daughter had not come to India with him on this trip.
“I’m regretting the choices I’ve made in life. I gave the prime of my youth to working for this country [the US] and now I feel like I’m not wanted.
“My daughter has spent her entire life in the US. I’m not sure how I’ll uproot my life from there and start all over in India.”
The H-1B is a work visa programme for people looking to work in the US in specialised fields and roles. Employers are able to sponsor professionals to get them into the country with a job offer required for the application.
According to government statistics, the greatest beneficiary of the programme the previous fiscal year was Amazon, followed by tech giants Tata, Microsoft, Meta, Apple and Google.
Another visa holder who was on holiday in Europe agreed there was confusion.
“We are yet to see how employers are thinking and how this will play out.
“From my understanding, the order is only for new H-1B visas. Immigration lawyers are still figuring it out and have advised us to go back.”
White House Press Secretary Karoline Leavitt posted on X clarifying some details including that it would not be an annual fee, just a one-off.
She wrote: “Those who already hold H-1B visas and are currently outside of the country right now will not be charged $100,000 to re-enter.
“H-1B visa holders can leave and re-enter the country to the same extent as they normally would.”
She added that the new fee would only apply to “new visas, not renewals, and not current visa holders”.
Business
Wegovy pill approved by US FDA for weight loss
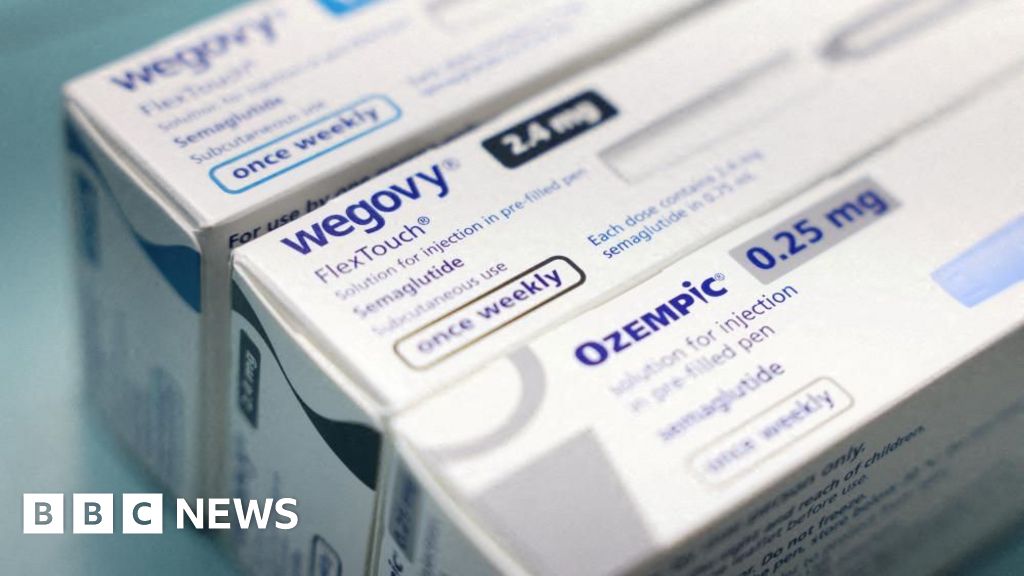
The US Food and Drug Administration (FDA) has approved a pill version of the weight-loss drug Wegovy, according to pharmaceutical giant Novo Nordisk.
It is the first pill of its kind to receive approval from the regulator, marking a new era for weight-loss drugs.
Wegovy’s Danish makers Novo Nordisk said the once-daily pill was a “convenient option” to the injectable and would provide the same weight loss as the shot. It comes after Wegovy was approved by the FDA specifically for weight loss.
Others like Ozempic, which has similar weight-loss effects, were primarily approved for the treatment of Type 2 diabetes.
The BBC has contacted the FDA for comment.
The Wegovy pill showed an average weight loss of 16.6% during Novo Nordisk’s trials, the firm said on Monday.
A third of around 1,300 participants experienced 20% or greater weight loss in the same trial, it added.
The pill is expected to be launched in the US in early January 2026.
“Patients will have a convenient, once-daily pill that can help them lose as much weight as the original Wegovy injection,” said Mike Doustdar, the firm’s chief executive.
The pill version of Wegovy could give Novo Nordisk’s sales a boost after a challenging year which saw its shares slide as it warned over its profits.
The company has faced intense competition in the weight-loss market from rival drugmakers like Eli Lilly.
Novo Nordisk’s shares rose by almost 10% in after-hours trade in New York after the announcement.
Business
FDA approves first GLP-1 pill for obesity from Wegovy maker Novo Nordisk

The logo of pharmaceutical company Novo Nordisk is displayed in front of its offices in Bagsvaerd, on the outskirts of Copenhagen, Denmark, Nov. 24, 2025.
Tom Little | Reuters
The U.S. Food and Drug Administration on Monday approved the first-ever GLP-1 pill for obesity from Wegovy maker Novo Nordisk, a landmark decision that health experts say could open up treatment access to more patients.
Novo Nordisk said it expects to launch the pill in early 2026. The Danish drugmaker said starting in early January, the starting dose of 1.5 milligrams will be available in pharmacies and via select telehealth providers with savings offers for $149 per month.
That’s the same price that cash-paying patients can access the starting dose of the pill on President Donald Trump’s direct-to-consumer website, TrumpRx, under a deal Novo Nordisk struck with his administration last month. Trump’s site also launches in January.
Novo Nordisk did not say how much higher doses of the drug would cost, but said additional information on coverage and savings options for eligible patients will be available at that time as well.
Shares of Novo Nordisk gained roughly 9% in extended trading Monday.
The FDA’s approval also clears the pill for use to reduce the risk of major cardiovascular events, such as death, heart attack or stroke, in adults with obesity and established cardiovascular disease, according to Novo Nordisk. That’s consistent with the approval label of the company’s blockbuster weight loss drug Wegovy, which shares the same active ingredient, semaglutide.
The move gives Novo Nordisk a head start over chief rival Eli Lilly, which is currently the dominant player in the market and is racing to launch its own obesity pill. Pills are the next battleground for the two drugmakers, which established the booming GLP-1 space that some analysts say could be worth roughly $100 billion by the 2030s.
Wall Street thinks there’s plenty of room for pills in the market, with Goldman Sachs analyst saying in August that pills could capture a 24% share — or around $22 billion — of the 2030 global weight loss drug market.
“What we’ve learned through years of research is that having an oral option really kind of opens up, activates and motivates different segments to seek treatment,” Dave Moore, Novo Nordisk’s executive vice president of U.S. operations, told CNBC ahead of the approval. “To have that conversation with their doctor to see if this is something that might be right for them.”
“That’s what we’re excited about — to be able to give people an option and make sure we have access and ease of access like we have been doing with our injections,” he continued.
The approval is based on a phase three trial that followed more than 300 adults with obesity but not diabetes.
In that study, a 25-milligram dose of Novo Nordisk’s oral semaglutide helped patients lose up to 16.6% of their weight on average after 64 weeks, according to results from the trial presented at a medical conference in 2024. That weight loss was 13.6% when the company analyzed all patients regardless of whether they stopped the drug.
The pill appears to be slightly more effective than an experimental oral drug from Eli Lilly, which is still waiting for FDA approval.
But unlike Novo Nordisk’s pill, Eli Lilly’s treatment is not a peptide medication. That means it is absorbed more easily by the body and does not require dietary restrictions. People who take Novo Nordisk’s pill have to wait 30 minutes before eating or drinking each day.
Moore said the prices of the pill get costs closer to what some people are paying for unapproved, compounded versions of branded GLP-1s, some of which are still being illegally mass marketed and sold in the U.S.
Patients flocked to the cheaper copycats when Ozempic and Wegovy were in short supply over the last two years due to skyrocketing demand, or if they didn’t have insurance coverage for the costly treatments. During FDA-declared shortages, pharmacists can legally make compounded versions of brand-name medications. But the agency earlier this year determined that the shortage of semaglutide is over, barring the practice in most cases.
“It continues to be alarming and disturbing for us,” Moore told CNBC, referring to illegitimate ingredients that are imported into the U.S. illegally and used by some compounding pharmacies to create copycat versions of GLP-1s.
This is breaking news. Please refresh for updates.
Business
Why Warner Bros. Discovery shareholders might opt for Paramount’s offer — and why they might not

Ted Sarandos, CEO of Netflix and David Zaslav, CEO of Warner Bros. Discovery.
Mario Anzuoni | Mike Blake | Reuters
Hours before Warner Bros. Discovery agreed to sell its studio and streaming assets to Netflix, Ted Sarandos, the co-CEO of Netflix, called WBD CEO David Zaslav to inform him Netflix wouldn’t be bidding any higher.
WBD shareholders now have a chance to call Sarandos’ bluff.
WBD shareholders have until Jan. 8 to tender their shares to Paramount for $30 in cash, though that deadline may be artificial. Paramount can extend it all the way to WBD’s annual meeting, which hasn’t been set yet but this year took place June 2.
If Paramount acquires 51% of outstanding WBD shares, it would control the company, even though the WBD board already agreed to sell the company’s studio and streaming assets to Netflix. Both Netflix and Paramount can use the coming days and weeks to speak with WBD shareholders to gauge whether they’d like to take Paramount’s offer or stick with the board’s recommendation to sell to Netflix.
To tender or not to tender, that is the question. There are sound arguments for both sides. The decision also presents a game theory element for shareholders who may simply want a bidding war rather than caring about the right buyer.
To tender
There are two overarching reasons why a shareholder might tender their holdings to Paramount.
The first is if the investor believes Paramount’s $30-per-share, all-cash offer for the entirety of WBD is more valuable than Netflix’s $27.75-per-share bid for just the Warner Bros. film studio and HBO Max streaming business. The second is a belief that tendering shares is the best way to force a bidding war between Netflix and Paramount.
A shareholder could decide Paramount’s current offer is better than Netflix’s if they think it has a higher likelihood of regulatory approval or if they believe Discovery Global — the portfolio of linear cable networks including CNN, TNT, Discovery, HGTV and TBS that’s set to be spun out — will have minimal value as a publicly traded company.
Paramount Skydance CEO David Ellison told CNBC earlier this month he values Discovery Global at $1 per share, given his prediction on the likely multiple (2 times earnings before interest, taxes, depreciation and amortization) at which it will trade based on current valuations for similar linear cable networks. If WBD doesn’t agree to sell the entire company to Paramount, it plans to split Discovery Global out as its own publicly traded entity in mid-2026.
Paramount’s argument is that $30 per share is already greater than Netflix’s $27.75-per-share offer plus $1 for Discovery Global.
David Ellison, CEO of Paramount Skydance, exits following an interview at the New York Stock Exchange (NYSE) in New York City, U.S., December 8, 2025.
Brendan Mcdermid | Reuters
Paramount’s bid is also all cash, while Netflix’s bid includes 16% equity with a so-called “collar,” which means shareholders won’t know exactly how much Netflix stock they’ll actually receive until the deal closes.
As for regulatory approval, Paramount has played up arguments that a combined Netflix and HBO Max streaming business would be anticompetitive. Netflix has more than 300 million global paying customers. The idea of the largest streamer buying HBO Max has already raised concerns with politicians, including President Donald Trump, who said there may be a “market share” issue with a Netflix deal.
While Paramount would combine Paramount+ with HBO Max, Paramount+ has about 80 million subscribers, presenting less of a risk to competition.
The second, more nuanced argument to tender is to maximize upside even if the assets ultimately go to Netflix.
Ellison has already made it known Paramount’s $30-per-share offer isn’t best and final. Tendering could cause Netflix to come back with a higher offer, which may then prompt Paramount to raise its bid as well.
GAMCO Investors chairman and CEO Mario Gabelli told CNBC earlier this month “the notion of Company A and Company B having a bidding war — that’s what we like as part of the free market system.”
He added last week that while he was previously leaning toward tendering his shares to Paramount, “the most important part is to keep it in play.”
Not to tender
Other shareholders may believe, in contrast, that not tendering is the best way of jumpstarting a bidding war. If Paramount sees that it’s not getting traction with shareholders as the annual meeting gets closer, it may raise its bid to get more shareholders on board.
There are additional reasons not to tender. Shareholders may want the Netflix and Discovery Global equity portion of the Netflix proposal.
In a WBD filing last week, the company said a mystery “Company C” proposed to acquire Discovery Global and its 20% stake in WBD’s streaming and studios business for $25 billion in cash. That bid was rejected by the WBD board as “not actionable.”
Still, the mystery bid suggests there may be an interested buyer in all of Discovery Global if it gets spun out, which could result in far more than $1 per share, according to Rich Greenfield, an analyst at LightShed Partners. That’s a good reason not to tender, he said, because it makes the Netflix offer much more valuable than Paramount’s bid.
Ensuring WBD splits Discovery Global is also the safe play for shareholders in case regulators block a Paramount-WBD merger, Greenfield said. Since the Paramount deal is for all of WBD, including CNN, Ellison’s bid — which includes roughly $24 billion from Middle Eastern sovereign funds — may run into regulatory and political hurdles, Greenfield noted.
“You want the split to happen,” Greenfield said in an interview. “If the Paramount deal doesn’t get regulatory approval, now you’ve prevented the split from happening. You’re stuck in 2027 with declining cable networks, and you haven’t spun them off. Does the U.S. really want a company funded by more Middle Eastern money than money from the Ellisons owning CNN?”
‘Where’s Poppa?’
WBD’s board has argued part its reasoning for rejecting Paramount’s $30-per-share bid was its concern with financing, noting more funding comes from Middle Eastern sovereign wealth funds than the Ellison family, which has committed about $12 billion.
Paramount altered the terms of its deal Monday to help address funding concerns. Oracle founder Larry Ellison, the father of David and one of the world’s five wealthiest people, agreed to provide “an irrevocable personal guarantee of $40.4 billion of the equity financing for the offer and any damages claims against Paramount,” should the existing financing fall through, Paramount said in a statement.
Paramount also said Monday it will publish records confirming the Ellison family trust “owns approximately 1.16 billion shares of Oracle common stock and that all material liabilities of the Ellison family trust are publicly disclosed.” Paramount has said the family trust will backstop the financing. WBD’s board had previously argued the trust is an “opaque entity,” preferring a direct commitment from the Ellisons.
Notably, even with the Monday announcement, the Ellisons haven’t increased their personal equity investment, which still stands at $12 billion. Internally, some WBD executives have cited the 1970 Carl Reiner movie “Where’s Poppa?” when speaking about the bid, according to a person familiar with the matter. WBD has pushed for the Ellisons to commit more personal money to the deal.
Still, a WBD shareholder may not care where the funding is coming from as long as it’s there. The three SWFs involved in the deal are the Saudi Arabian Public Investment Fund (PIF), Abu Dhabi’s L’imad Holding Company and the Qatar Investment Authority (QIA). The PIF and QIA, in particular, are known institutions that have contributed billions of dollars to other U.S.-based deals.
-

 Business1 week ago
Business1 week agoStudying Abroad Is Costly, But Not Impossible: Experts On Smarter Financial Planning
-

 Business1 week ago
Business1 week agoKSE-100 index gains 876 points amid cut in policy rate | The Express Tribune
-

 Fashion5 days ago
Fashion5 days agoIndonesia’s thrift surge fuels waste and textile industry woes
-

 Business5 days ago
Business5 days agoBP names new boss as current CEO leaves after less than two years
-

 Sports1 week ago
Sports1 week agoJets defensive lineman rips NFL officials after ejection vs Jaguars
-
 Tech1 week ago
Tech1 week agoFor the First Time, AI Analyzes Language as Well as a Human Expert
-

 Tech5 days ago
Tech5 days agoT-Mobile Business Internet and Phone Deals
-

 Entertainment1 week ago
Entertainment1 week agoPrince Harry, Meghan Markle’s 2025 Christmas card: A shift in strategy




