Business
Nandini Products To Get Cheaper: Karnataka Milk Federation Slashes Prices From Tomorrow

New Delhi: Starting Monday, September 22, the latest GST reforms announced after the 56th GST Council meeting will come into effect. Ahead of this rollout, ‘Nandini’—the popular milk and dairy brand from the Karnataka Milk Federation (KMF) has updated prices on 21 of its products.
From ghee and cheese to ice creams, chocolates, cookies, and cakes, consumers can expect a 7 per cent to 10 per cent cut in packaged dairy product prices across packs ranging from 80 grams to 1 kilogram. (Also Read: What You Need To Know About GST 2.0 As Tax Cuts Kick In On Monday)
Simpler Tax Structure With Just Two Main Slabs
The GST reforms aim to boost consumption, make industries more competitive, and reduce costs for middle-class families. As part of the changes, the number of GST slabs has been cut from four to just two—5 per cent and 18 per cent—making the tax system easier to follow for everyone. However, sin goods such as high-end SUVs and tobacco products will continue to attract a much higher 40 per cent GST. (Also Read: Big News For Households: GST Bachat Utsav Begins Tomorrow, Says PM Modi)
List of 21 Products with Revised Nandini Prices
Ghee (Pouch), 1000 ml – Old MRP: Rs 650 | New MRP: Rs 610
Butter – Unsalted, 500 gm – Old MRP: Rs 305 | New MRP: Rs 286
Paneer, 1000 gm – Old MRP: Rs 425 | New MRP: Rs 408
Goodlife Milk, 1000 ml – Old MRP: Rs 70 | New MRP: Rs 68
Cheese – Mozzarella Diced, 1 kg – Old MRP: Rs 480 | New MRP: Rs 450
Cheese – Processed, 1 kg – Old MRP: Rs 530 | New MRP: Rs 497
Ice Creams – Vanilla Tub, 1000 ml – Old MRP: Rs 200 | New MRP: Rs 178
Ice Cream – Family Pack Vanilla, 5000 ml – Old MRP: Rs 645 | New MRP: Rs 575
Ice Cream – Chocolate Sundae, 500 ml – Old MRP: Rs 115 | New MRP: Rs 102
Ice Creams – Mango Naturals, 100 ml – Old MRP: ₹35 | New MRP: ₹31
Savouries, 180 gm – Old MRP: Rs 60 | New MRP: Rs 56
Muffins, 150 gm – Old MRP: Rs 50 | New MRP: Rs 45
Cakes, 200 gm – Old MRP: Rs 110 | New MRP: Rs 98
Aqua (Water), 1000 ml – Old MRP: Rs 20 | New MRP: Rs 18
Payasa Mix, 200 gm – Old MRP: Rs 90 | New MRP: Rs 80
Jamoon Mix, 200 gm – Old MRP: Rs 80 | New MRP: Rs 71
Badam Powder, 200 gm – Old MRP: Rs 120 | New MRP: Rs 107
Coconut Cookies, 100 gm – Old MRP: Rs 35 | New MRP: Rs 31
Splass Whey Drinks, 200 ml – Old MRP: Rs 10 | New MRP: Rs 9.5
Bounce, 200 ml – Old MRP: Rs 15 | New MRP: Rs 15 (no change)
Rice Crispy Milk Choco, 80 gm – Old MRP: Rs 65 | New MRP: Rs 58
Business
Amazon blocks 1,800 job applications from suspected North Korean agents

A top Amazon executive has said the US technology giant has blocked more than 1,800 job applications from suspected North Korean agents.
North Koreans tried to apply for remote working IT jobs using stolen or fake identities, Amazon’s chief security officer Stephen Schmidt said in a LinkedIn post.
“Their objective is typically straightforward: get hired, get paid, and funnel wages back to fund the regime’s weapons programs,” he said, adding that this trend is likely to be happening at scale across the industry, especially in the US.
Authorities in the US and South Korea have warned about Pyongyang’s operatives carrying out online scams.
Amazon has seen a nearly one-third increase in job applications from North Koreans in the past year, said Mr Schmidt in his post.
He said the operatives typically work with people managing “laptop farms” – referring to computers based in the US that are run remotely from outside of the country.
The firm used a combination of artificial intelligence (AI) tools and verification by its staff to screen job applications, he said.
The strategies used by such fraudsters have become more sophisticated, Mr Schmidt said.
Bad actors are hijacking dormant LinkedIn accounts using leaked credentials to gain verification. They target genuine software engineers to appear credible, he said, urging firms to report suspicious job applications to the authorities.
Mr Schmidt warned employers to look out for indicators of fraudulent North Korean job applications, including incorrectly formatted phone numbers and mismatched education histories.
In June, the US government said it had uncovered 29 “laptop farms” that were being operated illegally across the country by North Korean IT workers.
They used stolen or forged identities of Americans to help North Korean nationals get jobs in the US, said the Department of Justice (DOJ).
It also indicted US brokers who had helped secure jobs for the North Korean operatives.
In July, a woman from Arizona was sentenced to more than eight years in jail for running a laptop farm to help North Korean IT workers secure remote jobs at more than 300 US companies.
The DOJ said the scheme generated more than $17m (£12.6m) in illicit gains for her and Pyongyang.
Business
Wegovy pill approved by US FDA for weight loss
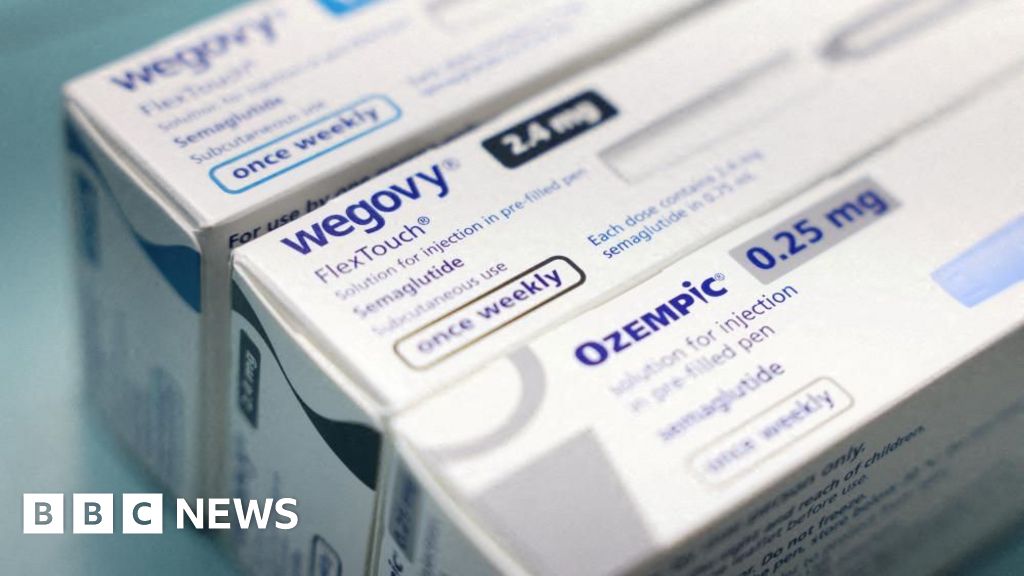
The US Food and Drug Administration (FDA) has approved a pill version of the weight-loss drug Wegovy, according to pharmaceutical giant Novo Nordisk.
It is the first pill of its kind to receive approval from the regulator, marking a new era for weight-loss drugs.
Wegovy’s Danish makers Novo Nordisk said the once-daily pill was a “convenient option” to the injectable and would provide the same weight loss as the shot. It comes after Wegovy was approved by the FDA specifically for weight loss.
Others like Ozempic, which has similar weight-loss effects, were primarily approved for the treatment of Type 2 diabetes.
The BBC has contacted the FDA for comment.
The Wegovy pill showed an average weight loss of 16.6% during Novo Nordisk’s trials, the firm said on Monday.
A third of around 1,300 participants experienced 20% or greater weight loss in the same trial, it added.
The pill is expected to be launched in the US in early January 2026.
“Patients will have a convenient, once-daily pill that can help them lose as much weight as the original Wegovy injection,” said Mike Doustdar, the firm’s chief executive.
The pill version of Wegovy could give Novo Nordisk’s sales a boost after a challenging year which saw its shares slide as it warned over its profits.
The company has faced intense competition in the weight-loss market from rival drugmakers like Eli Lilly.
Novo Nordisk’s shares rose by almost 10% in after-hours trade in New York after the announcement.
Business
FDA approves first GLP-1 pill for obesity from Wegovy maker Novo Nordisk

The logo of pharmaceutical company Novo Nordisk is displayed in front of its offices in Bagsvaerd, on the outskirts of Copenhagen, Denmark, Nov. 24, 2025.
Tom Little | Reuters
The U.S. Food and Drug Administration on Monday approved the first-ever GLP-1 pill for obesity from Wegovy maker Novo Nordisk, a landmark decision that health experts say could open up treatment access to more patients.
Novo Nordisk said it expects to launch the pill in early 2026. The Danish drugmaker said starting in early January, the starting dose of 1.5 milligrams will be available in pharmacies and via select telehealth providers with savings offers for $149 per month.
That’s the same price that cash-paying patients can access the starting dose of the pill on President Donald Trump’s direct-to-consumer website, TrumpRx, under a deal Novo Nordisk struck with his administration last month. Trump’s site also launches in January.
Novo Nordisk did not say how much higher doses of the drug would cost, but said additional information on coverage and savings options for eligible patients will be available at that time as well.
Shares of Novo Nordisk gained roughly 9% in extended trading Monday.
The FDA’s approval also clears the pill for use to reduce the risk of major cardiovascular events, such as death, heart attack or stroke, in adults with obesity and established cardiovascular disease, according to Novo Nordisk. That’s consistent with the approval label of the company’s blockbuster weight loss drug Wegovy, which shares the same active ingredient, semaglutide.
The move gives Novo Nordisk a head start over chief rival Eli Lilly, which is currently the dominant player in the market and is racing to launch its own obesity pill. Pills are the next battleground for the two drugmakers, which established the booming GLP-1 space that some analysts say could be worth roughly $100 billion by the 2030s.
Wall Street thinks there’s plenty of room for pills in the market, with Goldman Sachs analyst saying in August that pills could capture a 24% share — or around $22 billion — of the 2030 global weight loss drug market.
“What we’ve learned through years of research is that having an oral option really kind of opens up, activates and motivates different segments to seek treatment,” Dave Moore, Novo Nordisk’s executive vice president of U.S. operations, told CNBC ahead of the approval. “To have that conversation with their doctor to see if this is something that might be right for them.”
“That’s what we’re excited about — to be able to give people an option and make sure we have access and ease of access like we have been doing with our injections,” he continued.
The approval is based on a phase three trial that followed more than 300 adults with obesity but not diabetes.
In that study, a 25-milligram dose of Novo Nordisk’s oral semaglutide helped patients lose up to 16.6% of their weight on average after 64 weeks, according to results from the trial presented at a medical conference in 2024. That weight loss was 13.6% when the company analyzed all patients regardless of whether they stopped the drug.
The pill appears to be slightly more effective than an experimental oral drug from Eli Lilly, which is still waiting for FDA approval.
But unlike Novo Nordisk’s pill, Eli Lilly’s treatment is not a peptide medication. That means it is absorbed more easily by the body and does not require dietary restrictions. People who take Novo Nordisk’s pill have to wait 30 minutes before eating or drinking each day.
Moore said the prices of the pill get costs closer to what some people are paying for unapproved, compounded versions of branded GLP-1s, some of which are still being illegally mass marketed and sold in the U.S.
Patients flocked to the cheaper copycats when Ozempic and Wegovy were in short supply over the last two years due to skyrocketing demand, or if they didn’t have insurance coverage for the costly treatments. During FDA-declared shortages, pharmacists can legally make compounded versions of brand-name medications. But the agency earlier this year determined that the shortage of semaglutide is over, barring the practice in most cases.
“It continues to be alarming and disturbing for us,” Moore told CNBC, referring to illegitimate ingredients that are imported into the U.S. illegally and used by some compounding pharmacies to create copycat versions of GLP-1s.
This is breaking news. Please refresh for updates.
-

 Business1 week ago
Business1 week agoStudying Abroad Is Costly, But Not Impossible: Experts On Smarter Financial Planning
-

 Business1 week ago
Business1 week agoKSE-100 index gains 876 points amid cut in policy rate | The Express Tribune
-

 Fashion5 days ago
Fashion5 days agoIndonesia’s thrift surge fuels waste and textile industry woes
-

 Sports1 week ago
Sports1 week agoJets defensive lineman rips NFL officials after ejection vs Jaguars
-

 Business5 days ago
Business5 days agoBP names new boss as current CEO leaves after less than two years
-
 Tech1 week ago
Tech1 week agoFor the First Time, AI Analyzes Language as Well as a Human Expert
-

 Entertainment1 week ago
Entertainment1 week agoPrince Harry, Meghan Markle’s 2025 Christmas card: A shift in strategy
-

 Tech5 days ago
Tech5 days agoT-Mobile Business Internet and Phone Deals






