Business
Publishers fear AI summaries are hitting online traffic
Suzanne BearneTechnology Reporter
 Getty Images
Getty ImagesWhen actress Sorcha Cusack left the BBC drama Father Brown in January, it made headlines, including for the newspapers owned by Reach, among them The Mirror, and the Daily Express.
But the story did not generate the traction the Reach newspapers would have expected a year ago, or even at the start of the year.
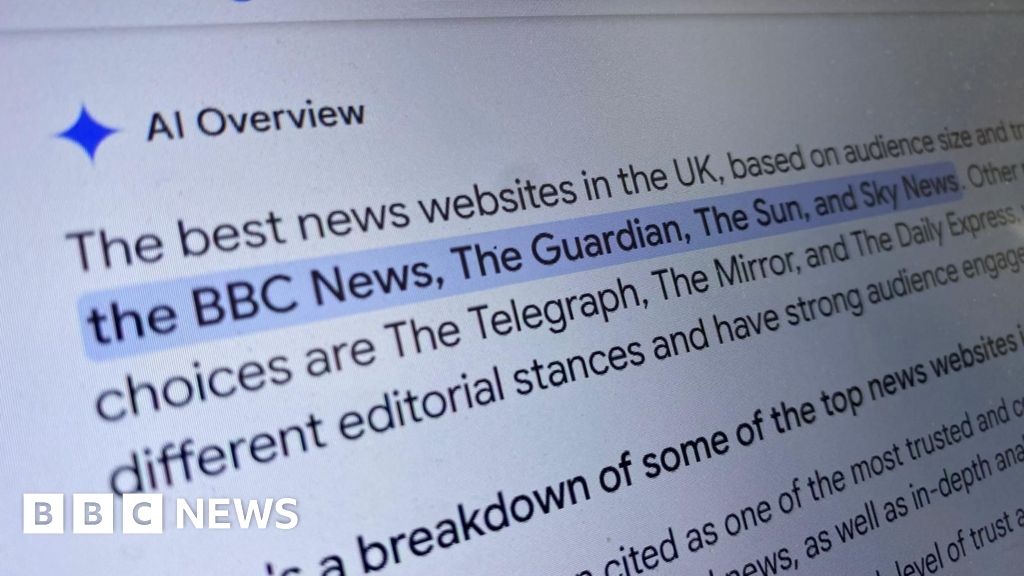
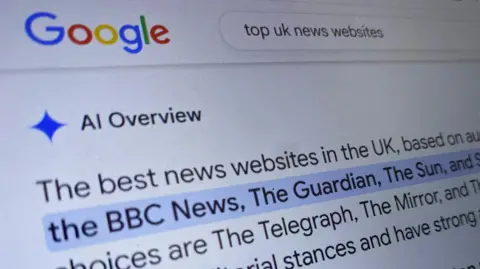
Reach put this down to AI Overviews (AIO) – the AI summary at the top of the Google results page.
Instead of clicking through to the story on a Reach newspaper site, readers were happy with the AI overview.
The feature is a concern for newspapers and other media publishers, who have already seen much of their advertising revenue siphoned off by social media.
In a tough market, readers coming via Google search is a valuable source of traffic.
“A major worry, backed by some individual datapoints, has been that AI overviews would lead to fewer people clicking through to the content behind them, with negative knock-on effects for publishers,” says Dr Felix Simon, research fellow in AI and news at the Reuters Institute for the Study of Journalism, University of Oxford.
He points out that it’s hard to know the scale of the problem, as Google does not publish data on click-through rates.
DMG Media, owner of MailOnline, Metro and other outlets, said AIO resulted in a fall in click-through-rates by as much as 89%, in a statement to the Competition and Markets Authority made in July.
It means publishers are not being fairly rewarded for their work, says David Higgerson, chief digital publisher at Reach.
“Publishers provide the accurate, timely, trustworthy content that basically fuels Google, and in return we get a click… that hopefully we can monetise to our subscription service.
“Now with Google Overviews it’s reducing the need for somebody to click through to us in the first place, but for no financial benefit for the publisher.”
“It’s another example of the distributor of information not being the creator of information but taking all the financial reward for it.”
There is also concern over Google’s new tool called AI Mode, which shows search results in a conversational style with far fewer links than traditional search.
“If Google flips onto full AI Mode, and there is a big uptake in that…that [will be] completely quite devastating for the industry,” says Mr Higgerson.
 Getty Images
Getty Images“We are definitely moving into the era of lower clicks and lower referral traffic for publishers,” says Stuart Forrest, global director of SEO digital publishing at Bauer Media.
“For most of the last decade Google has introduced more and more features into the SERP [Search Engine Results Page], which reduces the need for consumers to visit a website. That is the challenge that we as a sector face.”
Mr Forrest says he hasn’t noticed a drop in traffic across Bauer’s sites, which include brands Grazia and Empire, as a result of the overview feature. But that could change.
“I absolutely think that as time goes on, as consumers get used to these panels, it’s without doubt going to be a challenge. We are absolutely behaving as if we have to respond to that threat.”
In its defence, a Google spokesperson said: “More than any other company, Google prioritises sending traffic to the web, and we continue to send billions of clicks to websites every day.
In an August blog post, Google’s head of search Liz Reid said the volume of clicks from Google search to websites had been “relatively stable” year-over-year.
She also said the number of quality of clicks had improved slightly compared to a year ago – quality clicks are when a user does not immediately click back from the link.
“With AI Overviews, people are searching more and asking new questions that are often longer and more complex. In addition, with AI Overviews people are seeing more links on the page than before. More queries and more links mean more opportunities for websites to surface and get clicked,” she said in the blog.
Some in the publishing industry are turning to the courts for redress.
In July, a group of organisations including the Independent Publishers Alliance, tech justice non-profit Foxglove, and the campaign group Movement for an Open Web filed a legal complaint to the UK’s Competition and Markets Authority alleging that Google AI Overviews is using publishers’ content at a cost to the newspapers.
It is asking the CMA to introduce interim measures to prevent Google from “misusing” publisher content in AI-generated responses.
In the meantime publishers are trying to understand how to feature in AIO and hopeful win some click-throughs.
“Google doesn’t give us a manual on how to do it. We have to run tests and optimise copy in a way that doesn’t damage the primary purpose of the content, which is to satisfy a reader’s desire for information,” explains Mr Higgerson.
“We need to make sure that it’s us being cited and not our rivals,” says Mr Forrest. “Things like writing good quality content… it’s amazing the number of publishers that just give up on that.”
Like other publishers, Reach is looking at other ways to build traffic to its news platforms.
“We need to go and find where audiences are elsewhere and build relationships with them there. We’ve got millions of people who receive our alerts on WhatsApp,” Mr Higgerson says.
“We’ve built newsletters. It’s all about giving people what they want when they’re on our website and our brand, so the next time they’re looking, hopefully they aren’t going to a third party to get to us.”
Business
Nike tops earnings estimates but shares fall as China sales plunge, tariffs hit profits

A shopper carries Nike bags in San Francisco, California, US, on Wednesday, Dec. 17, 2025.
David Paul Morris | Bloomberg | Getty Images
Nike on Thursday posted quarterly earnings and revenue that topped Wall Street’s estimates, as strength in North America helped to offset a plunge in China sales.
The company’s stock slid more than 6% in extended trading Thursday, as investors digested the weakness in China and the sustained hit Nike is taking from higher tariffs.
Here’s what Nike reported for its second fiscal quarter of 2026, according to consensus estimates from LSEG:
- Earnings per share: 53 cents vs. 38 cents expected
- Revenue: $12.43 billion vs. $12.22 billion expected
The athletic apparel retailer said sales in North America rose 9% to $5.63 billion. But revenue in its Greater China market dropped 17% to $1.42 billion.
The sneaker company is just over a year into CEO Elliott Hill’s turnaround strategy, focusing on regaining its growth and market share, clearing out old inventory and investing in wholesale relationships.
“Fiscal year ’26 continues to be a year of taking action to rightsize our classics business, return Nike digital to a premium experience, diversify our product portfolio, deepen our consumer connection, strengthen our partner relationships and realign our teams and leadership,” Hill said on a call with analysts. “And I say we’re in the middle inning of our comeback.”
“We’re nowhere near our potential,” he added.
Hill said Nike’s improvements in its China market are “not happening at the level or the pace we need to drive wider change,” though he said the country remains one of the company’s most powerful long-term opportunities.
Nike expects fiscal third quarter revenues to fall by a low single digit percentage, with modest growth in North America. It also anticipates gross margins will drop 1.75 to 2.25 percentage points – including a 3.15 percentage point hit from tariffs.
The company said wholesale revenues climbed 8% to $7.5 billion during the quarter. But direct sales — which were a focus for Nike in the years before Hill took over and moved away from the strategy — fell 8% to $4.6 billion.
Nike has also been feeling the impact of tariff increases. It said Thursday that its gross margin decreased by 3 percentage points and inventories dropped 3% primarily due to higher tariffs.
The sneaker company has been reporting weakness in its Converse brand, too. In its first fiscal quarter, Nike said Converse sales dropped 27% – on Thursday, it reported a 30% drop in revenues for the sneaker brand.
Despite the weakness in some parts of Nike’s business, the company highlighted some areas of strength and new initiatives ahead. CFO Matt Friend said on the call that Nike.com posted its best Black Friday ever this year, partially driven by its Air Jordan “Black Cat” launch.
Nike also plans to launch a new footwear platform in January called Nike Mind, which aims to help athletes prepare for performance and competition, Hill said on the call.
Nike has been making larger internal changes under Hill.
Earlier this month, Nike underwent leadership changes to “remove layers,” according to Hill. Under its “Win Now” strategy, the company announced that Chief Commercial Officer Craig Williams would leave the sneaker giant.
Hill called the shakeup a move “about growth and offense.”
“Collectively, these changes amount to us eliminating layers and better positioning Nike to continue to have an impact the way only Nike can,” Hill said in a statement at the time.
Nike shares have dropped more than 13% this year as of Thursday’s close.
Business
SHANTI shields N-plants from safety oversight: Experts – The Times of India

NEW DELHI: The new nuclear energy bill, which was passed in Rajya Sabha by voice vote after a four-hour discussion while rejecting many amendments moved by opposition to send it to a parliamentary panel for scrutiny, marks a decisive shift in India’s nuclear governance, embedding safety oversight in law across the lifecycle of an atomic plant, unlike the existing framework that relied largely on executive discretion and post-accident accountability.Sustainable Harnessing of Nuclear Energy for Transforming India (SHANTI) Bill will allow private participation in India’s tightly controlled civil nuclear sector as the country seeks to meet its clean energy goals by 2047. As opposition raised safety and liability concerns, officials said it establishes a statutory safety regime that ensures continuous compliance rather than reliance on one-time permissions. It seeks to provide for a “pragmatic civil liability regime for nuclear damage and confer statutory status to Atomic Energy Regulatory Board (AERB)”.Officials said unlike the previous law – in which nuclear safety oversight was shaped largely by broad executive authority and administrative rules – SHANTI fundamentally recasts the framework by shifting to a “statutory, lifecycle-based regulatory regime”. Govt manages radiation risks and radioactive waste, but does not mandate separate safety authorisations or legally bind safety obligations to each phase of a nuclear plant’s life. AERB’s stage-wise consent process for construction, commissioning and operation existed only as an administrative practice. Civil Liability for Nuclear Damage (CLND) Act, 2010 further reinforced a post-accident approach by focusing on compensation and insurance rather than prevention.“These laws (Atomic Energy Act and CLND Act) treated safety primarily as a post-damage responsibility, rather than a proactive governance requirement,” said an official. SHANTI separates “permission to operate” from “permission to operate safely”, requiring both a licence and an independent safety authorisation. Any activity involving radiation exposure risk – including construction, operation, transport, storage, decommissioning, or waste management – will now require explicit safety approval.It also consolidates regulation, enforcement, civil liability and dispute resolution within a single statute, reducing legal complexity and compliance uncertainty. “It grants a clear statutory authority to AERB to inspect facilities, investigate incidents, issue binding directions, and suspend or cancel operations that do not meet safety standards. Regulatory action is no longer dependent on executive discretion. Accident prevention is significantly enhanced by legally recognising serious risk situations as nuclear incidents, even without actual damage,” said the official. Core functions such as fuel enrichment, spent-fuel reprocessing, and heavy water production will remain exclusively under Centre’s control.Anujesh Dwivedi, partner at Deloitte India, said continuing with the existing legal framework would make it difficult for nuclear energy to replace thermal power in the long run. “Over decades, India added only about 8GW of nuclear capacity. Scaling this up to 100GW by 2047- and potentially 300GW or more by 2070 – required major reforms, which these regulations seek to address,” he said.Meanwhile, PM Modi said passing of the bill marks a “transformational moment for our technology landscape”.
Business
American Airlines no longer lets basic economy flyers earn miles

American Airlines
Grant Baldwin | Getty Images
American Airlines customers flying on basic economy fares will no longer earn frequent flyer miles or points toward elite status, the carrier said this week.
“We routinely evaluate our fare products to remain competitive in the marketplace. Customers who purchase a Basic Economy ticket on December 17, 2025 and beyond will not earn AAdvantage miles or Loyalty Points towards AAdvantage status,” it said. “Basic Economy customers will continue to receive one free personal item and one free carry-on bag, free snacks, soft drinks and in-flight entertainment.”
Elite loyalty members will still be eligible for first-class upgrades on domestic flights if they’re on basic economy tickets, an American spokeswoman told CNBC.
Basic economy tickets are airlines’ cheapest but most restrictive fares, rolled out across the industry over the past decade. Generally, they do not allow customers to change their tickets without fees or pick their seats in advance.
The move comes as airlines across the board have been chasing customers who are willing to spend more to fly. American has fallen behind large rivals Delta Air Lines and United Airlines in the post-Covid luxury travel boom.
American’s change, posted earlier by X user JonNYC, follows a similar policy by competitor Delta Air Lines, which said travelers on its Delta Main Basic, or basic economy tickets, wouldn’t receive Delta SkyMiles.
United Airlines does allow its MileagePlus loyalty program members to earn miles on basic economy tickets, but it has a different limitation: Basic economy customers on most flights aren’t allowed to bring a carry-on bag.
American had the same restriction after it launched basic economy fares but backpedaled in 2018.
Southwest Airlines this year launched its first no-frills basic fares that stipulate those customers will board last and get a seat assignment at check-in and earn miles at a lower rate than more expensive fares.
-

 Business5 days ago
Business5 days agoHitting The ‘High Notes’ In Ties: Nepal Set To Lift Ban On Indian Bills Above ₹100
-

 Politics1 week ago
Politics1 week agoTrump launches gold card programme for expedited visas with a $1m price tag
-

 Business1 week ago
Business1 week agoRivian turns to AI, autonomy to woo investors as EV sales stall
-

 Sports1 week ago
Sports1 week agoPolice detain Michigan head football coach Sherrone Moore after firing, salacious details emerge: report
-

 Fashion1 week ago
Fashion1 week agoTommy Hilfiger appoints Sergio Pérez as global menswear ambassador
-

 Business1 week ago
Business1 week agoCoca-Cola taps COO Henrique Braun to replace James Quincey as CEO in 2026
-

 Tech1 week ago
Tech1 week agoGoogle DeepMind partners with UK government to deliver AI | Computer Weekly
-

 Sports1 week ago
Sports1 week agoU.S. House passes bill to combat stadium drones







