Tech
Amazon’s 2 Best-Selling Espresso Machines are on Sale Before Prime Day

Hi. it turns out Prime Day is only really a suggestion on the calendar. A lot of brands like to kick out early Prime Day deals before getting lost in the big din of Prime Big Deal Days on October 7-8. (See here for early Prime Day deals on laptops, earbuds, and more.)
The two most popular espresso machines on Earth—as judged by Amazon sales, anyway–are both on sale for $100 off this week, ahead of Amazon Prime Day’s October reprise. These are a couple of the best early Prime Day Deals.
$100 off Ninja Luxe Cafe Premier Before Prime Day
The most exciting deal of the pair is probably the Ninja Luxe Cafe Premier ($500), on sale for the lowest price we’ve ever seen it.
When Ninja announced it was jumping into the semiautomatic espresso market, I didn’t know quite what to make of it. But Ninja seems to have applied its general flair for multipurpose machines to this beautifully beginner-friendly espresso machine with a 25-setting conical burr grinder, a built-in scale (thank you), and options for cold brew and drip coffee.
WIRED contributing reviewer Tyler Shane was likewise skeptical of Ninja’s first espresso device when it arrived but ended up loving the excellent milk steaming and the fact that this Ninja grinds espresso shots by weight. (Why doesn’t everybody?) She also appreciated the reasonable price—a price that’s even more reasonable ahead of Prime Day.
$100 off Breville Barista Express
Breville’s Barista Express ($600) semiautomatic espresso machine has been Amazon’s best-selling espresso machine for years—so long it’s hard to remember a time when it wasn’t the top-selling pick.
Why’s it so popular? It’s a Goldilocks thing—a mix of accessible price, Breville’s excellent reputation for customer service on high-ticket items, and beautiful ease of use on a semiautomatic machine with a built-in grinder that makes full-flavored, well-extracted espresso. This $100 discount isn’t quite the lowest price we’ve seen on it—it’s been down to $550 before—but it’s a very good price.
And besides, this Breville has the merit of being a tried-and-true machine. WIRED reviewer Julian Chokkattu has been pulling shots from his Barista Express for six years now, and it’s still going strong. No wonder the Express has been among WIRED’s top espresso machine picks for ages.
Tech
DHS Opens a Billion-Dollar Tab With Palantir

The Department of Homeland Security struck a $1 billion purchasing agreement with Palantir last week, further reinforcing the software company’s role in the federal agency that oversees the nation’s immigration enforcement.
According to contracting documents published last week, the blanket purchase agreement (BPA) awarded “is to provide Palantir commercial software licenses, maintenance, and implementation services department wide.” The agreement simplifies how DHS buys software from Palantir, allowing DHS agencies like Customs and Border Protection (CBP) and Immigration and Customs Enforcement (ICE) to essentially skip the competitive bidding process for new purchases of up to $1 billion in products and services from the company.
Palantir did not immediately respond to a request for comment.
Palantir announced the agreement internally on Friday. It comes as the company is struggling to address growing tensions among staff over its relationship with DHS and ICE. After Minneapolis nurse Alex Pretti was shot and killed in January, Palantir staffers flooded company Slack channels demanding information on how the tech they build empowers US immigration enforcement. Since then, the company has updated its internal wiki, offering few unreported details about its work with ICE, and Palantir CEO Alex Karp recorded a video for employees where he attempted to justify the company’s immigration work, as WIRED reported last week. Throughout a nearly hourlong conversation with Courtney Bowman, Palantir’s global director of privacy and civil liberties engineering, Karp failed to address direct questions about how the company’s tech powers ICE. Instead, he said workers could sign nondisclosure agreements for more detailed information.
Akash Jain, Palantir’s chief technology officer and president of Palantir US Government Partners, which works with US government agencies, acknowledged these concerns in the email announcing the company’s new agreement with DHS. “I recognize that this comes at a time of increased concern, both externally and internally, around our existing work with ICE,” Jain wrote. “While we don’t normally send out updates on new contract vehicles, in this moment it felt especially important to provide context to help inform your understanding of what this means—and what it doesn’t. There will be opportunities we run toward, and others we decline—that discipline is part of what has earned us DHS’s trust.”
In the Friday email, Jain suggests that the five-year agreement could allow the company to expand its reach across DHS into agencies like the US Secret Service (USSS), Federal Emergency Management Administration (FEMA), Transportation Security Administration (TSA), and the Cybersecurity and Infrastructure Security Agency (CISA).
Jain also argued that Palantir’s software could strengthen protections for US citizens. “These protections help enable accountability through strict controls and auditing capabilities, and support adherence to constitutional protections, especially the Fourth Amendment,” Jain wrote. (Palantir’s critics have argued that the company’s tools create a massive surveillance dragnet, which could ultimately harm civil liberties.)
Over the last year, Palantir’s work with ICE has grown tremendously. Last April, WIRED reported that ICE paid Palantir $30 million to build “ImmigrationOS,” which would provide “near real-time visibility” on immigrants self-deporting from the US. Since then, it’s been reported that the company has also developed a new tool called Enhanced Leads Identification & Targeting for Enforcement (ELITE) which creates maps of potential deportation targets, pulling data from DHS and the Department of Health and Human Services (HHS).
Closing his Friday email to staff, Jain suggested that staffers curious about the new DHS agreement come work on it themselves. “As Palantirians, the best way to understand the work is to engage on the work directly. If you are interested in helping shape and deliver the next chapter of Palantir’s work across DHS, please reach out,” Jain wrote to employees, who are sometimes referred to internally as fictional creatures from The Lord of the Rings. “There will be a massive need for committed hobbits to turn this momentum into mission outcomes.”
Tech
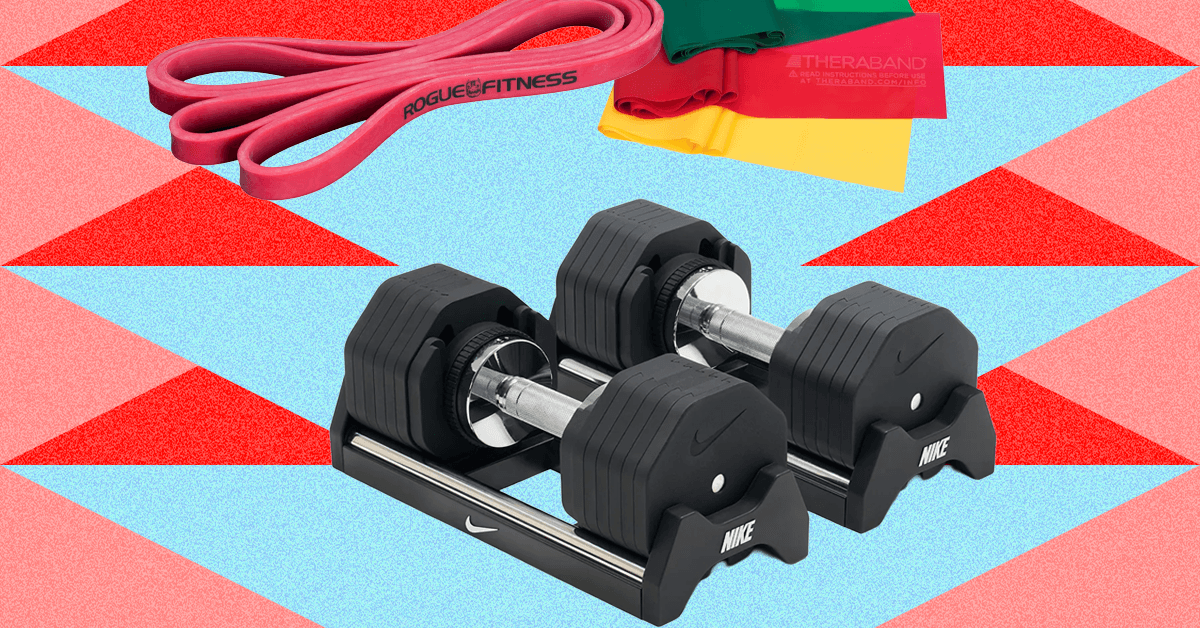
If You’re Building a Home Gym, Start With Dumbbells and a Yoga Mat
To join or not to join a gym: That is the question. If you opt out of building a home gym, you can join a club and have access to more weights and machines. Friends and classes motivate you to keep coming, and that monthly bill keeps you disciplined. On the other hand, gym memberships are steep, workouts can get hijacked by bullies, and going to the gym is an additional commute.
My gym tardiness, however, will likely catch up to me. One of the most consistent messages from health and fitness experts today is that lifting weights has immeasurable benefits. Strength training allows us to keep doing the things we love well into our advanced years. It reduces blood sugar, lowers blood pressure, burns calories, and reduces inflammation. A recent review of studies in the British Journal of Sports Medicine by Harvard Medical School found that strength training is linked to lower risk for cardiovascular disease, diabetes, and cancer and provides a 10 to 17 percent lower overall risk of early death.
But you don’t need all the time and money in the world to have a great home gym. Reviews editor Adrienne So and I have been slowly adding to our existing, minimalist home gyms in our living rooms and garage—a roughly 10- by 10-foot patch in our basements and living rooms. There’s a ton of equipment out there, but for maximum results, I asked two physical therapists—Grace Fenske at Excel North Physical Therapy and Performance and Samuel Hayden at Limit Less Physical Therapy—for their recommendations.
Here’s a PT-recommended guide for an ultrasimple setup that will keep you pumped and motivated. Don’t see anything you like? Don’t forget to check out our existing guides to the Best Running Shoes, the Best Fitness Trackers, or the Best Walking Pads.
Jump To
Adjustable Dumbbells
Yes, these are very pricey. But people outgrow their small dumbbells very quickly, and if you bite the bullet early, adjustable dumbbells take up a lot less space than individual dumbbell or kettlebell sets. The Nüobell adjustable dumbbells required 38 patents and allow users to increase weight in increments of five pounds all the way up to 80 with a twist of the handle. Each dumbbell set replaces 32 individual dumbbells. In a cramped space, that’s a game changer.
The way that both Steph’s Nüobells and my Nike adjustable dumbbells work is that the full barbell fits into a cradle. (You can also mount the barbells in a stand.) When the user twists the handle to five pounds, the aluminum bar with grooves will grab onto the first hollowed-out plate, which is 2.5 pounds on each side of the barbell. With each subsequent turn of the handle the bar will pick up heavier weight in increments of five pounds. A safety hook at the bottom of the cradle ensures the barbell weight must be locked in place before lifting.
I like my Nike dumbbells because the end of the dumbbell is flat, which means I can rest it on its end on my thigh without putting a divot in my leg. Also, the plates aren’t round. If you have a big round dumbbell on the floor, or especially in your garage, it will find the nearest incline and roll away on top of a house pet or child. You can still take individual plates out of the rack if you need them for leverage under your heel or for mobility exercises. Whichever one you choose, though, both Steph and I recommend getting a floor stand to decrease strain on your back. —Adrienne So
Tech
This AI Tool Will Tell You to Stop Slacking Off
I’ve tested a lot of software tools over the years designed to block distractions and keep you focused. None of them work perfectly, mostly because of context.
Reddit, for example, is something I should generally avoid during the workday, so I tend to block it—this is a good decision for me overall. The problem is that sometimes the only place I can find a particular piece of information online is in a Reddit thread, meaning that to get that information I need to turn off my distraction-blocking tool. Then I inevitably end up down some kind of rabbit hole.
This is the exact problem Fomi, a macOS distraction-blocking tool, is built to solve. The application asks you what you’re working on, then watches everything you do on your Mac desktop—every app you open—and uses AI to analyze what’s on your screen. The tool can tell, from context, whether you’re using a particular website productively or as a distraction.
Zach Yang, part of the team behind the app, tells me on Discord he dreamed up the app after talking with a friend who was studying for an MBA. “He needed YouTube for study videos, so web/app blockers didn’t work, and once he was watching, recommendations would often pull him away,” Yang says. “That’s when I started thinking about using AI to solve this. I built a small prototype to test whether current models were capable of distinguishing distraction from actual work, and the results were good enough that I decided to turn it into a real project.”
Fomi offers a three-day free trial. If you decide you like it, subscription plans cost $8 per month. However, since the tool uploads screenshots of your desktop to an AI model in the cloud, there are privacy concerns you will need to weigh before deciding if a tool like this is right for you.
Watch This Space
I’ve been trying out this application for a couple of days. The first time you launch it, you’re asked what you do day-to-day and what kind of tools you use to do it. Then, when it’s time to focus, you tell the software what you’re working on and which tools you plan to use while doing it.
As you work, a green dot and a timer appear at the top of the screen, surrounding your MacBook’s notch. If you switch to a potentially distracting application, the dot changes to yellow. If you start engaging in things that are clearly distractions, the dot turns red and an animated tomato splats across the screen. You’ll see a custom message telling you to get to work—the app calls out your specific distraction.
Courtesy of Justin Pot
-

 Business1 week ago
Business1 week agoGold price today: How much 18K, 22K and 24K gold costs in Delhi, Mumbai & more – Check rates for your city – The Times of India
-

 Business7 days ago
Business7 days agoTop stocks to buy today: Stock recommendations for February 13, 2026 – check list – The Times of India
-

 Politics1 week ago
Politics1 week agoIndia clears proposal to buy French Rafale jets
-

 Fashion1 week ago
Fashion1 week agoIndia’s PDS Q3 revenue up 2% as margins remain under pressure
-

 Fashion7 days ago
Fashion7 days ago$10→ $12.10 FOB: The real price of zero-duty apparel
-

 Fashion1 week ago
Fashion1 week agoWhen 1% tariffs move billions: Inside the US apparel market repricing
-

 Tech1 week ago
Tech1 week agoElon Musk’s X Appears to Be Violating US Sanctions by Selling Premium Accounts to Iranian Leaders
-

 Tech2 days ago
Tech2 days agoRakuten Mobile proposal selected for Jaxa space strategy | Computer Weekly





















